This is a promotional email sent on behalf of Novartis Pharmaceuticals UK Ltd, intended for UK healthcare professionals only.
A link to the prescribing information and adverse event reporting for ENTRESTO® (sacubitril/valsartan) is found here.
|
|
|
|
|
ENTRESTO is indicated in adult patients for the treatment of symptomatic chronic heart failure with reduced ejection fraction.4
|
Dear Healthcare professional,
Despite advances in treatment, the burden of heart failure (HF) in the UK is increasing with around 690,000 people on their GP’s heart failure register and what is thought to be over 900,000 affected.5,6,7 HF is estimated to account for around 2% of the NHS budget – about £2 billion.7
|
|

|
Hospitalisations comprise 70% of HF costs
of the NHS8
|

|
Hospital admissions due to HF are projected to rise by 50% over the next 25 years9
|

|
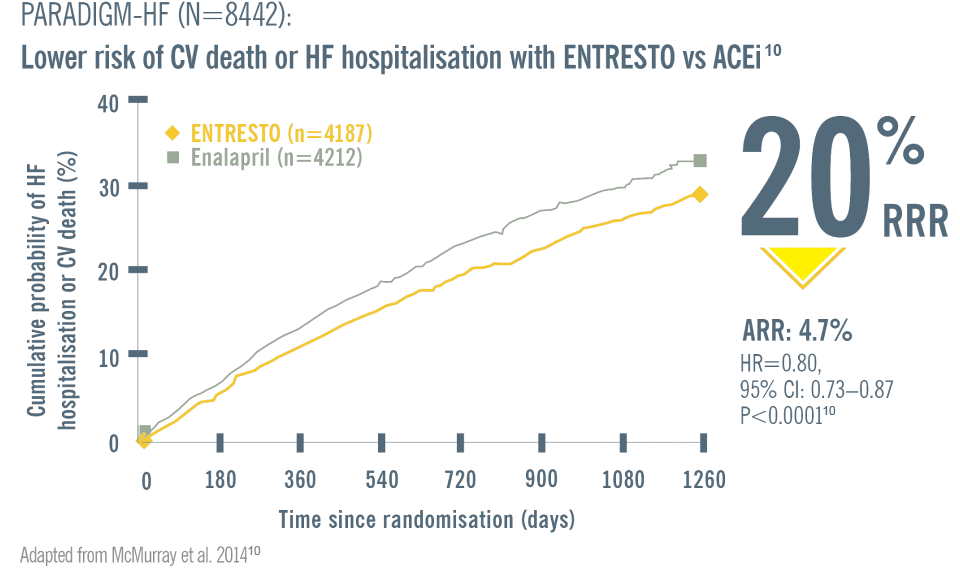
Patients on ENTRESTO experienced improved QoL, reduced hospitalisations and decreased mortality vs ACEi (enalapril)§1-4,10-15
QoL based on post hoc analysis.§16
|
|
|
|
For full safety and dosing information please refer to the Summary of Product Characteristics (SmPC). Click here for GB and here for NI.
|
|
Help reduce the burden on the NHS by prescribing ENTRESTO today for your symptomatic chronic HFrEF patients.
|
|
|
|
|
There is a 6x greater risk of death in the first month after just one HF hospitalisation in comparison with non-hospitalised patients17,18
|
Start ENTRESTO to help patients stay out of
hospital and improve QoL vs ACEi (enalapril)4
QoL based on post hoc analysis.§16
|
|
|
Starting ENTRESTO earlier in the disease trajectory reduces HF hospitalisations, which may save hospital budgets and alleviate system burden.‖19
|
|
|
|
Initiating ENTRESTO is as simple as starting with an ACEi (enalapril).4,20 Flexible starting doses are available tailored to your patients’ needs, with titration similar to ACEi (enalapril).¶4,20
|

|
|
•
|
ENTRESTO demonstrated superior efficacy with a safety profile generally comparable to ACEi (enalapril) in chronic HFrEF patients.10 In PARADIGM-HF, hypotension was reported for ENTRESTO 740 (17.6%) vs enalapril 506 (12%).
|
|
•
|
The most commonly reported adverse reactions during treatment with sacubitril/valsartan were hypotension (17.6%), hyperkalaemia (11.6%) and renal impairment (10.1%). Angioedema was reported in patients treated with sacubitril/valsartan (0.5%).‖4
|
|
|
|
|
| If you would like a personalised discussion around the practicalities of starting ENTRESTO, in addition to receiving patient support materials for those prescribed ENTRESTO, please contact the team through the below email address.
|
Kind regards
The Novartis UK ENTRESTO Team
ENTRESTO.uk@novartis.com
|
|
| * CaReMeUK Partnership recommends offering sacubitril/valsartan or ACEi (or ARB if intolerant to ACEi) + BB and MRA as first-line treatment for chronic HFrEF patients.1 |
| † 2021 ESC Guidelines recommend sacubitril/valsartan as part of cornerstone HFrEF therapy, together with BBs, MRAs, and SGLT2is.2 |
| ‡ The 2022 AHA/ACC/HFSA Guideline recommends ARNI as the preferred first-line treatment option for chronic HFrEF patients with NYHA Class II to III symptoms.3 This is a US guideline and does not inform clinical practice. |
| § In a post-hoc analysis of this study, the differences in outcomes of the KCCQ between treatment arms were assessed. Patients in the ENTRESTO group had significantly better adjusted change scores in 7/10 physical and social activities vs enalapril. The largest improvements over enalapril were in household chores (adjusted change score difference, 2.35 [95% CI, 1.19–3.50], P<0.001) and sexual relationships (adjusted change score difference, 2.72 [95% CI: 0.97–4.46], P=0.002); both persisted through 36 months (overall change score difference, 1.69 [95% CI: 0.78–2.60], P<0.001; and 2.36 [95% CI: 1.01–3.71], P=0.001, respectively).16 |
| ‖ ENTRESTO (n=4,187) showed superior reductions in chronic HFrEF hospitalisations and CV death compared to enalapril (n=4,212) [death from CV causes or first hospitalisation for worsening of HF: ARR=4.7%; NNT=21, p<0.001]19 |
| ¶ For the primary endpoint, composite of CV death or first HF hospitalisation, ENTRESTO was superior to enalapril (P<0.001).12 |
|
|
| Abbreviations:
|
|
ACC, American College of Cardiology; ACEi, angiotensin-converting enzyme inhibitor; AHA, American Heart Association; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CaReMe UK, Cardio-Renal-Metabolic Partnership UK; CI, confidence interval; CV, cardiovascular; ESC, European Society of Cardiology; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SGLT2i, sodium-glucose co-transporter-2 inhibitor; QoL, quality of life. |
|
|
| References:
|
|
1. |
CaReMe UK Partnership HF algorithm. Available at: https://www.britishcardiovascularsociety.org/resources/careme (Accessed August 2023). |
|
2. |
McDonagh TE, et al. Eur Heart J 2021;42(36):3599–3726. |
|
3. |
Heidenreich P A, et al. Circulation 2022;145(18):e895–e1032. |
|
4. |
ENTRESTO Summary of Product Characteristics. Electronic medicines compendium website, UK. Available at: https://www.medicines.org.uk/emc/product/7751/smpc. (Accessed August 2023). |
|
5. |
Conrad N, et al. Lancet 2018;391:572–580. |
|
6. |
British Heart Foundation. UK Factsheet. 2023. Available at: https://www.bhf.org.uk/what-we-do/our-research/heart-statistics (Accessed August 2023). |
|
7. |
National Institute for Health and Care Excellence. Resource impact report: Chronic heart failure in adults: diagnosis and management (NG106). 2018. Available at: https://www.nice.org.uk/guidance/ng106/resources/resource-impact-report-pdf-6537494413. (Accessed August 2023). |
|
8. |
House of Commons Library. Debate Pack Quality of life for patients with heart failure, 2021. https://researchbriefings.files.parliament.uk/documents/CDP-2021-0034/CDP-2021-0034.pdf. (Accessed August 2023). |
|
9. |
National Institute for Health and Care Excellence. Enhancing the Quality of Heart Failure Care https://www.nice.org.uk/Media/Default/sharedlearning/Enhancing%20quality-heart%20failure%20V11_WEB.PDF. (Accessed August 2023). |
|
10. |
McMurray JJ, et al. N Engl J Med 2014;371(11):993-1004. |
|
11. |
Desai AS, et al. JAMA 2019;322(11):1077-1084. |
|
12. |
Velazquez EJ, et al. N Engl J Med 2019;380(6):539-548. |
|
13. |
Morrow DA, et al. Circulation 2019;139(19):2285-2288. |
|
14. |
Ambrosy AP, et al. J Am Coll Cardiol 2020;76(9):1034-1048. |
|
15. |
Claggett B, et al. N Engl J Med 2015;373(23):2289-2290. |
|
16. |
Chandra A et al. JAMA Cardiol 2018;3(6):498-505. |
|
17. |
Solomon SD, et al. Circulation 2007;116(13):1482–1487. |
|
18. |
Okumura N, et al. Circulation 2016;133(23):2254–2262. |
|
19. |
Gaziano TA et al. JAMA Cardiol 2020;5(11):1236-1244. |
|
20. |
Enalapril Summary of Product Characteristics. Electronic medicines compendium website, UK. Available at: https://www.medicines.org.uk/emc/product/561/smpc. (Accessed August 2023). |
|
|
|
|
© Copyright 2023 Novartis Pharmaceuticals UK LTD
UK | August 2023 | 276662
|
 |
|
|
|
|
|
|
|
|