View the web version of this email.
Please add news@medicalimi.com to your address book to ensure that our emails reach your inbox |
|
This is a promotional email sent on behalf of Novartis and is intended for UK Healthcare Professionals only.
KESIMPTA®▼ (ofatumumab) is indicated for the treatment of adults with relapsing forms of multiple sclerosis
with active disease defined by clinical or imaging features.1
Prescribing information for KESIMPTA® (ofatumumab) and adverse event reporting details can be found here.
|
|
|
|
| KESIMPTA delivers consistent mean IgG levels for up to 5 years and with new safety signals1,2
|
|
|
|
|
|
|

|
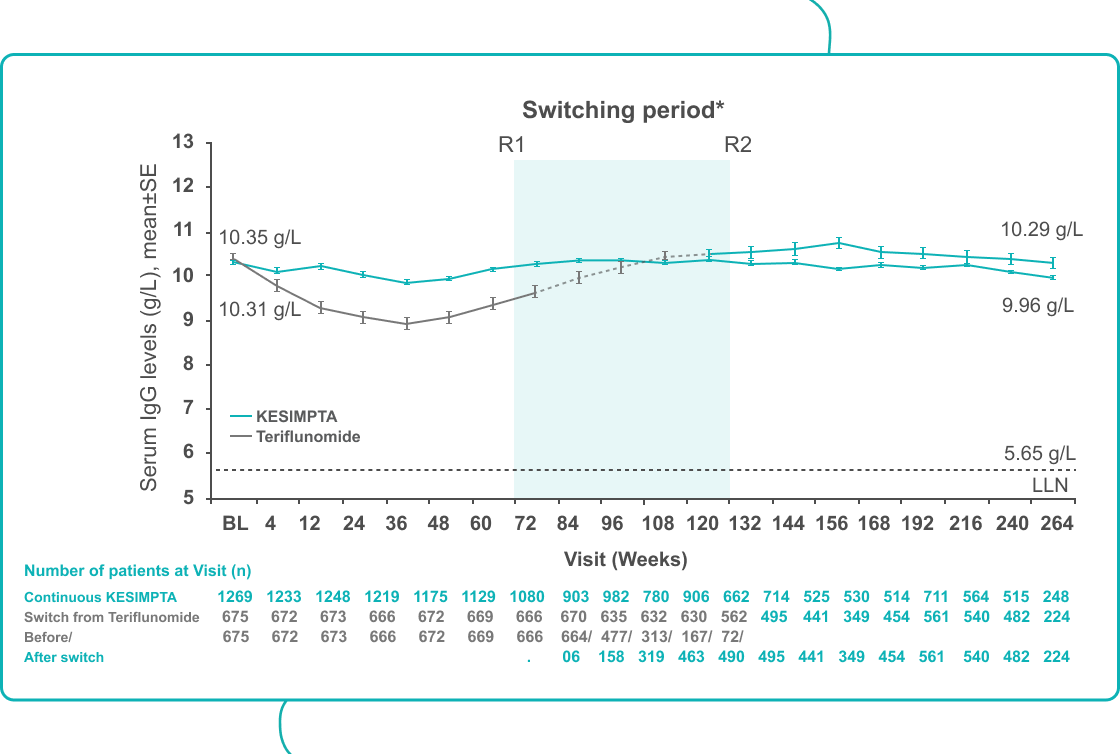
| In ALITHIOS, mean serum IgG levels remained stable and above the LLN in KESIMPTA-treated patients for up to 5 years†2
|
|
|
|
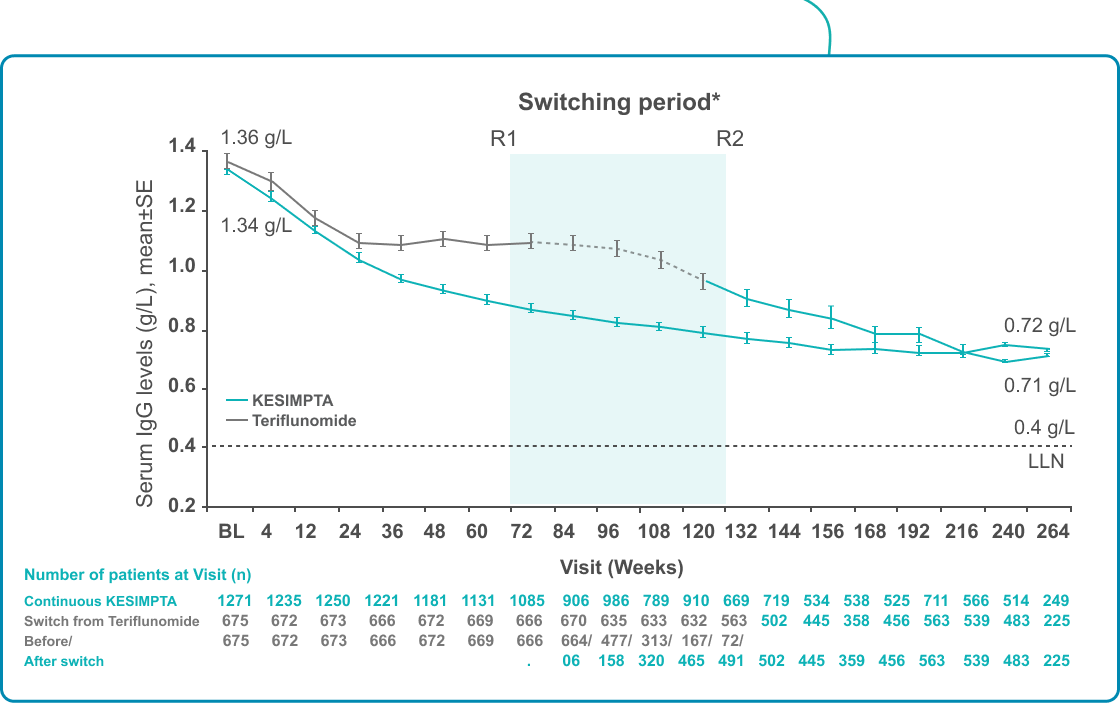
| A decrease in IgM levels was observed for KESIMPTA-treated patients but remained above the LLN for up to 5 years†2
|
|
|
|
|
|

|
|
|
|
• |
The most common AEs were infections (COVID-19 [30.3%], nasopharyngitis [19%], URTI [12.8%] and UTI [12.7%]).2 |
|
• |
There was no association between decreased IgG/IgM levels and the risk of serious infection.2 |
|
• |
The incidence of serious infections in KESIMPTA-treated patients was low (1.63 EAIR per 100 PYs).2 |
|
• |
Overall infection rates are comparable to teriflunomide during the core study up to 30 months. Infection rates continued to remain stable for up to 5 years in the single arm extension study ALITHIOS.1,2 |
|
|
|
|
|
|
|

|
|
|
| KESIMPTA is the first subcutaneous, fully human B-cell targeting mAb3,4
|
|
|
| It is thought to work by selectively binding to sites on both the small and large extracellular loops of CD204 |
|
|
|
|
|
|
| KESIMPTA is a targeted and precisely delivered B-cell therapy1,5-7
|
|
|
| Preclinical evidence shows SC delivery preferentially targets B cells in the lymph nodes1,5,6 and spares B cells in the spleen, which may help maintain immune function7 |
|
|
|
|
|
|
| The recommended dose of KESIMPTA is 20 mg SC1
|
|
|
| This was chosen through dose modelling based on B-cell depletion results and Phase 2 data8-10 |
|
|
|
|
|
|
|
|
|
Footnotes: *Switching period refers to the patients started with teriflunomide and not applicable to the patients with KESIMPTA in core period; for teriflunomide/KESIMPTA group, data from first dose of teriflunomide until last dose of KESIMPTA plus 100 days/analyses cut-off date have been used; R1: The first patient with first treatment-emergent assessment in KESIMPTA period after switching to KESIMPTA (72 weeks); R2: The last patient with last treatment emergent assessment in teriflunomide period before switching to KESIMPTA (120 weeks); for all pooled analyses, a fixed value of LLN (using ALITHIOS study reference) was used: IgG: 5.65 g/L and IgM: 0.4 g/L; †Based on results from the ALITHIOS extension study, data cut-off: Sept 2022.
Abbreviations: AE=adverse event; BL=baseline; EAIR=exposure adjusted incident rate; HR=hazard ratio; Ig=immunoglobulin; LLN=lower limit of normal; MS=multiple sclerosis; PY=patient-years; SC=subcutaneous; SE=standard error; URTI= upper respiratory tract infection; UTI=urinary tract infection.
References: 1. KESIMPTA® Summary of Product Characteristics (GB and NI); 2. Cohen JA, et al. Presented at the American Association of Neurology (AAN) Annual meeting; 22-27 April (Boston) 2023. P8.004; 3. National Multiple Sclerosis Society. Medications. Available from: https://www.nationalmssociety.org/Treating-MS/Medications [Accessed: January 2024]; 4. Gupta IV, Jewell RC. Ann NY Acad Sci. 2012;1263:43-56; 5. Torres JB, et al. Presented at the AAN Annual meeting; 4-10 May 2019; Philadelphia, US. P2.2-052; 6. Huck C, et al. J Neuroimmune Pharmacol. 2019;14(4):709 719; 7. Theil D, et al. Front Immunol. 2019;10:1340; 8. Sorensen PS, et al. Neurology. 2014;82:573 581; 9. Bar-Or A, et al. Neurology. 2018;90:e1805-e1814; 10. Savelieva M, et al. Presented at the AAN Annual meeting; 22-28 April 2017; Boston, US. P5.348.
|
|
|
|
|
|
This email message is sent on behalf of Novartis Pharmaceuticals UK Ltd Registered Office Second Floor of The WestWorks Building, White City Place, 195 Wood Lane, London W12 7FQ. Registered number 00119006.
Confidentiality notice: This email message, including any attachments, is for the sole use of the intended recipient(s) and may contain confidential and privileged information. Any unauthorised review, use, disclosure or distribution is prohibited. If you are not the intended recipient, please contact the sender by reply email and destroy all copies of the original message. |
|
|
|
| UK | January 2024 | 307685 |
|
|
|
 |
This email was sent to email@email.co.uk from news@medicalimi.com
Unsubscribe | Receive in Plain Text |
Copyright © ICR (UK) Limited t/a International Medical Information (IMI).
Registered in the UK Company no. 05894351
2nd Floor, Nucleus House, 2 Lower Mortlake Road, Richmond, Surrey, TW9 2JA
Contact info@medicalimi.com. All rights reserved. Click here for details of our Data Protection and Privacy Policy 2018 |
|
|
