This is a promotional email for UK Healthcare Professionals,
sent on behalf of Takeda UK Ltd.
Prescribing Information and Adverse Event reporting can be found at the end of this email.
Problems viewing this email? View it in your browser. |

|
|
|
LOOKS LIKE DEPRESSION.
|
SOUNDS LIKE
ANXIETY.
|
|
MIGHT BE ADHD.1,2
|
|
|
|
|
|
|
| ADHD is a common and treatable disorder, but is underdiagnosed in adults.3
Diagnosis of ADHD in adulthood is made difficult by having overlapping symptoms,1,2 with more than 1 in 6 patients with a mental health condition also having ADHD.4* |
| *2,284 adult patients with a previously diagnosed mental health condition consented, of whom 1,986 completed the study. ADHD was diagnosed using the Diagnostic Interview for ADHD in Adults, version 2.0 (DIVA). Based on the DIVA, and applying DSM-IV-TR or DSM-5 criteria, 15.8 % (95 % CI 14.2 %-17.4 %) or 17.4 % (95 % CI 15.7 %-19.0 %) of patients were diagnosed with
ADHD, respectively.4 |
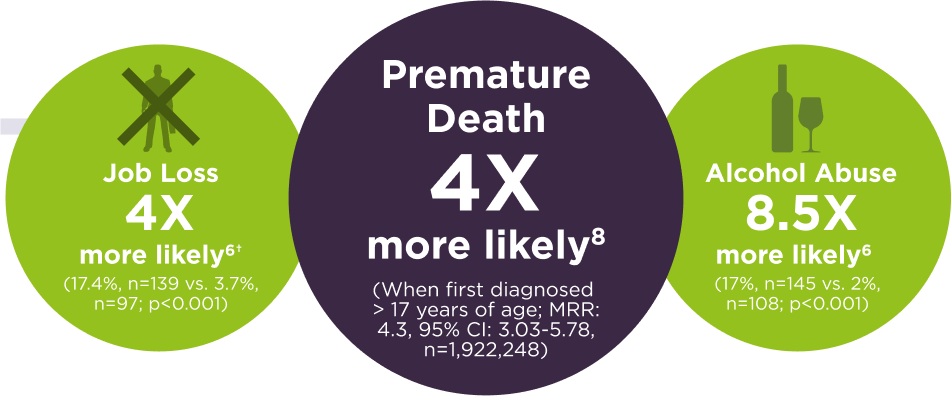
Adults with ADHD have worse outcomes in
life vs. healthy controls5-8 |
 |
Treat their ADHD and help reduce the
impact of ADHD on their lives5‡ |
|
|
|
|
†Difference taken from general population control data.
‡Impact is defined here as an increased likelihood of negative life events/trends, or decreased likelihood of positive life events/trends.5 |
|
|
|
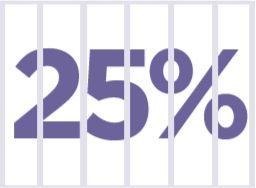
25% of the adult prison population have ADHD,§ which is 10 times higher than the global prevalence.9
|
|
|
|
|
Appropriate treatment of ADHD resulted in up to 41% reduction‖ in criminality and could alleviate the symptoms of substance use disorders and psychiatric comorbidities.3,10
|
§Based on meta-analysis of 42 prisons.9
‖The crime rate was reduced by 32% among men and 41% among women (p<0.001) during treatment periods.10
|
|
|
How can Elvanse Adult help your adult
patients with ADHD?
|
 |
Prodrug technology for consistent symptom control.11‑13 |
 |
Taken once-daily for 14-hour core symptom control.13,14 |
 |
Significantly improved executive function¶ and quality of life vs. placebo at 10 weeks.15,16 |
 |
The most frequently reported adverse reactions (≥1/10) with Elvanse Adult are decreased appetite, dry mouth, headache and insomnia.14 |
| ¶As measured by the validated, self-reported Brown Attention-Deficit Disorder Scale (BADDS) |
|
|
Clicking this button will take you to a Takeda resource on
BMJ.com for ADHD/Elvanse Adult.
|
|
|
|
|
Lisdexamfetamine is recommended by NICE as a first‑line treatment for adults with ADHD.17
|
|
When prescribing stimulants for ADHD, think about modified-release once-daily preparations for the following reasons:
|
|
|
|
|
| • |
Reducing stigma (as there is no need to take medication in the workplace) |
|
| • |
Reducing problems of storing and administering controlled drugs at school |
|
| • |
Avoiding the risk of stimulant misuse and diversion associated with immediate-release preparations |
|
| • |
The pharmacokinetic profiles of the stimulants |
|
|
|
|
|
|
Elvanse Adult is indicated as part of a comprehensive treatment programme for attention deficit/hyperactivity disorder
in adults.14
Elvanse Adult is not indicated in all adult patients and the decision to use the medicinal product must take into consideration the profile of the patient, including a thorough assessment of the severity and chronicity of the patient’s symptoms, the potential for abuse, misuse or diversion, and clinical response to any previous pharmacotherapies for the treatment of ADHD.14
Elvanse Adult treatment must be under the supervision of a specialist in behavioural disorders.14
CI=confidence interval; MRR=mortality rate ratio; NICE=National Institute for Health and Care Excellence
|
| References |
| 1. |
Searight HR et al. Am Fam Physician 2000;62(9):2077−86. |
| 2. |
Kooij JJS et al. J Atten Disord 2012;16:3S−19S. |
| 3. |
Ginsberg Y et al. Prim Care Companion CNS Disord. 2014;16(3): PCC.13r01600. Available at:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4195639/ (Accessed October 2023). |
| 4. |
Deberdt W et al. BMC Psychiatry 2015;15:242. |
| 5. |
Chang Z et al. JAMA Psychiatry 2017;74(6):597–603. |
| 6. |
Barkley RA et al. ADHD in Adults. New York: Guilford Press, 2008. |
| 7. |
Biederman J et al. J Clin Psychiatry 2006;67(4):524–40. |
| 8. |
Dalsgaard S et al. Lancet 2015;385:2190–6. |
| 9. |
Young S et al. Psychol Med. 2015;45(2):247–258. doi: 10.1017/S0033291714000762. |
| 10. |
Lichtenstein P et al. N Engl J Med 2012;367(21):2006–14. |
| 11. |
Ermer JC et al. Clin Drug Investig. 2016;36:341–56. |
| 12. |
Ermer JC et al. J Clin Pharmacol. 2010;50:1001–10. |
| 13. |
Wigal T et al. Behav Brain Funct. 2010;6:34. |
| 14. |
Elvanse® Summary of Product Characteristics. Available at https://www.emcpi.com/grp/47 (Accessed October 2023). |
| 15. |
Adler LA et al. J Clin Psychiatry 2013;74:694–702. |
| 16. |
Adler LA et al. BMC Psychiatry 2013;13:253. |
| 17. |
NICE guidelines NG87 (2018). Attention deficit hyperactivity disorder: diagnosis and management. Available at nice.org.uk/guidance/ng87 (Accessed October 2023). |
|
|
| Click here for Prescribing Information |
Job Code: C-APROM/GB/NS/0609
Date of Preparation: October 2023
|
 |
|
|
©2023 Takeda UK Limited. All rights reserved. Takeda® and the Takeda Logo® are registered trademarks of Takeda Pharmaceutical Company Limited. Takeda UK Limited, 1 Kingdom Street, London, W2 6BD, United Kingdom.
|
This email has been forwarded to you by Data4NHS, on behalf of Takeda UK Ltd.
Manage your Data4NHS profile by submitting your changes HERE.
View our privacy policy HERE.
UNSUBSCRIBE. |
|
|